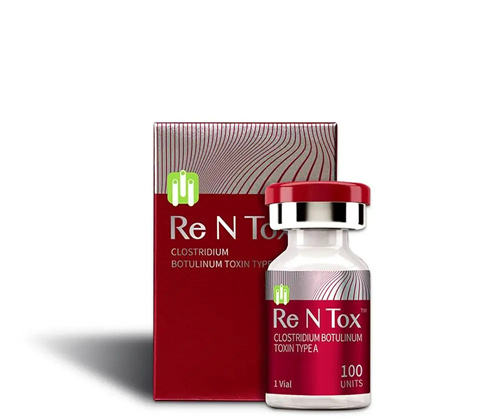
Description
ReNTox ™ 200 units is an innovative drug (botulinum toxin) type A Premium Class, developed by the South Korean company Pharma Research Bio. It is presented as a lyophilized white powder for injection in a colorless transparent vial.
BENEFITS:
1) 3D effect: The patient should observe the effect from the 3rd day after administration, the duration of action is 6-8 months
2) Composition: the improved formula of the preparation does not contain: proteins, albumin, gelatin compared to other botulinum toxins.
3) Diffusion: has the lowest diffusion of all currently known botulinum toxins.
EFFECTIVENESS:
- ReNTox guarantees high efficiency.
- The fluctuation in potency is tightly controlled in the range of 191-215 units
- Rentox provides a precise and uniform effect at the time of injection, guaranteeing high patient satisfaction with the preparation
INDICATIONS:
1-Correction of mimic wrinkles
2-Spasm of the eyelids. 3-Spasm of the half-face
4-cervical dystonia (spastic torticollis)
5-Focal spasticity of the wrist and hand in post-stroke patients
6-paralytic strabismus (strabismus)
7-local muscle spasms in cerebral palsy in children 2 years of age and older
Form of issue: 1 bottle / 200 pcs.
Storage conditions: Store at a temperature of 2 ° C to 8 ° C




Reviews
There are no reviews yet.